BioEnthesis®

the first-of-its-kind
biphasic interpositional
allograFt FOR rotator cuff repair
biphasic interpositional
allograFt FOR rotator cuff repair

Why Cancellous Bone is Better than Cortical Bone
Learn nowClinical Challenge
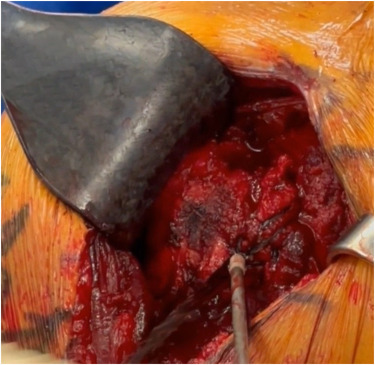
Too many rotator cuff repairs result in re-tear. Why? Healing relies on reincorporation of tendon into the bone at the enthesis. See what the literature says about the enthesis:
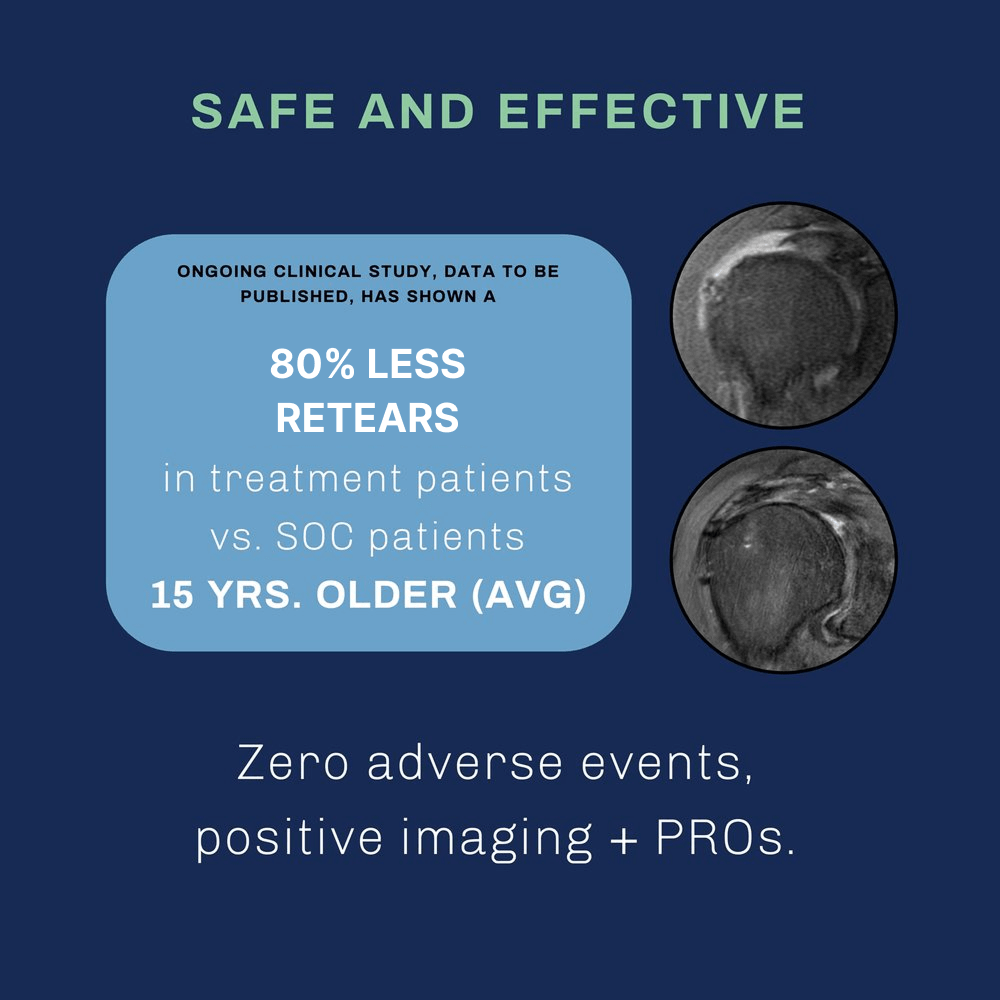
B.U.C. THE TREND OF ROTATOR CUFF RE-TEAR
WITH BIOENTHESIS UNDER THE CUFF.
BACKED BY SCIENCE
OUTSTANDING CLINICAL PROFILE
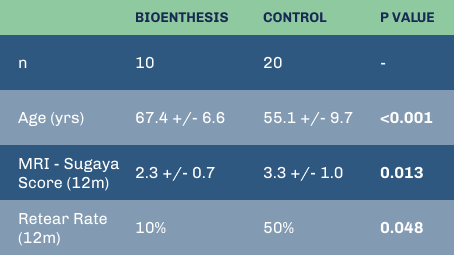
PUBLICATION PENDING; DATA AVAILABLE UPON REQUEST
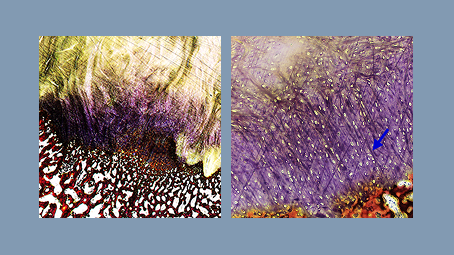
Sharpey fiber formation +
reduced inflammation
in ovine model.
reduced inflammation
in ovine model.
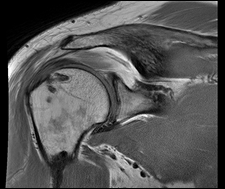
Despite significantly older patients, BioEnthesis showed fewer retears and significantly better MRIs.
Publications
Indication for Use:
BioEnthesis is indicated to provide a matrix for the repair or reconstruction of the bone of the enthesis within the rotator cuff.
Contraindications:
BioEnthesis is contraindicated for use in any patient in whom soft and hard tissue implants are contraindicated. This includes any pathology that would limit the blood supply and compromise healing, or evidence of a current infection. BioEnthesis is contraindicated where the allograft is intended as structural support in load-bearing bone, and articular cartilage surfaces.
INOVA Patient Set
Set of 2 patients out of the Northern Virginia area with postoperative scores and MRIs